BioGenware’s Speculet Seeks to Improve the Patient Experience
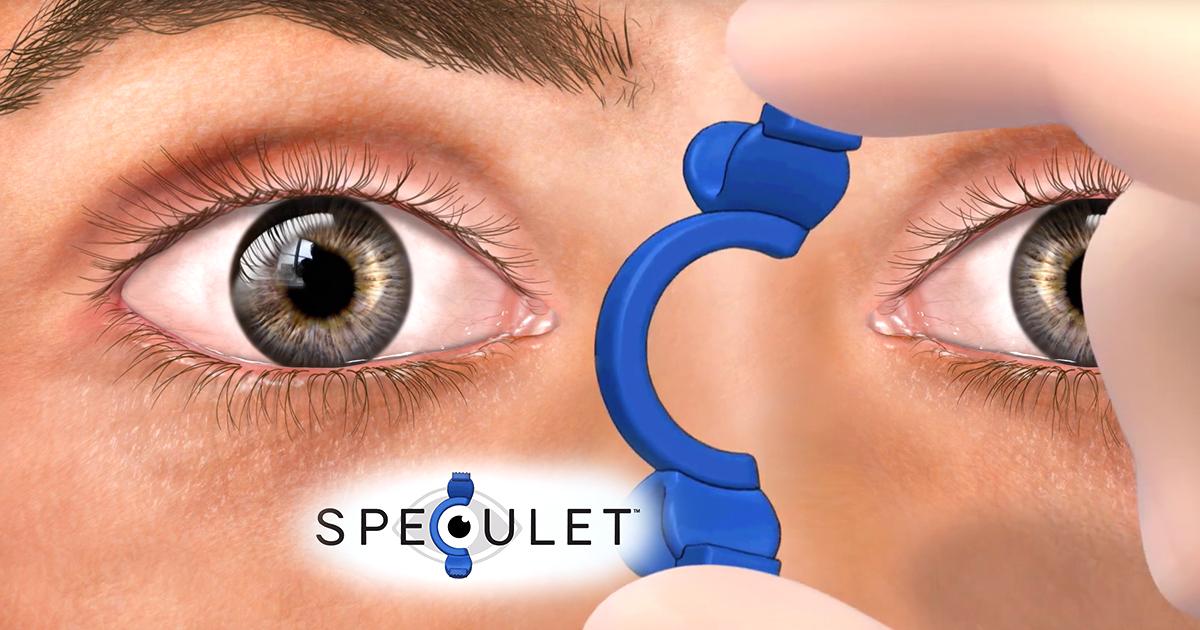
For those undergoing intravitreal injections (and there are about 22 million of these performed yearly worldwide), a common complaint is that the speculum is uncomfortable, sometimes even downright painful. Jeffrey Gross, MD, co-founder & managing partner, BioGenware, and founder & president of the Carolina Retina Center, developed a device along with co-founder and partner John Lowsky, Jr., BSME, that hopes to alleviate both the pain and discomfort of the traditional speculum. (Dr. Gross noted he personally performs between 4,000 and 5,000 intravitreal injections a year.)
The Speculet is “a newly designed reinvented speculum to effectively open the eyelids and provide a comfortable patient experience for intravitreal injections,” according to the company. Although pricing has not been finalized BioGenware estimates potential sales of 300,000 Speculets in 2020 (at around $1 million, or at a cost of under $4 per packaged Speculet), increasing to 475,000 units in 2021 (about $1.6 million in sales). The company predicts that an improved patient experience in the medical office will drive demand for the comfortable Speculet. The profit from just one additional premium anti-VEGF injection may pay for an entire day’s use of the Speculet, given a range of 15-40 injections per typical clinic day, Dr. Gross said.
The Speculet solution
“Current speculums hurt, they’re cold, they stretch the lids, and they can cause bruising of the lids. In some patients they’re just difficult to insert,” Dr. Gross said. “There is also a lot of maintenance involved, with repeatedly disinfecting and re-packaging hundreds of speculums.”
Dr. Gross decided a solution was needed that will be comfortable for patients; be something physicians, nurses, and techs can all easily insert and remove; and is inexpensive enough to be disposable, being delivered sterile and thrown away after one use. The Speculet—a disposable resin lid speculum designed to maximize patient comfort and physician access for both nasal and temporal injections in either eye—is placed between the lids using a novel rotational insertion technique and unique finger tabs.
“Patients find this much more comfortable than the sudden stretching of the lids using the traditional metal and wire speculum,” Dr. Gross said.
The Speculet was initially designed for intravitreal injection of anti-VEGF agents and steroids, and, with use of a smaller version, for infant exams and retinopathy of prematurity (ROP) injections. Many infants are injected bilaterally for ROP; newer guidelines issued in 2018 require the use of a sterile speculum and instruments for each exam, Dr. Gross added.
In its current form it can also be useful for indirect ophthalmoscopy retinal laser requiring scleral depression. However the company is developing it as a platform device, and there are accessories that can be attached. For example, there is a stabilizer that can be used instead of a cotton tip or a forceps to hold the eye still for aqueous paracentesis and other treatments.
Modifications of the device may be useful in ultra-widefield imaging to reduce lash artifacts, and in corneal and cataract procedures to provide a comfortable and disposable option eliminating the risks of cross contamination. The current Speculet can both hold a commercial macular vitrectomy lens and keep the lids open. This “may be advantageous for shorter vitrectomy cases such as macular pucker, macular holes and floaters,” Dr. Gross said.
The novel device has attracted quite a bit of attention, as it took first place at The Winning Pitch Challenge at this year’s American Society of Retinal Specialists’ annual meeting. An honorarium of $10,000 was given for winning first place in the Challenge, which BioGenware has used to finalize tooling of the injection mold and for final testing of resins.
Beyond the retina, Dr. Gross said the platform application may be useful in anterior segment surgeries as well, in particular, corneal and cataract procedures (as it can improve comfort and reduce patient anxiety).
The company website reports that as a result of the Challenge win, retina colleagues have asked the BioGenware design team to CAD and prototype their inventions. BioGenware is in talks with individuals and companies to provide distribution channels worldwide.
