Takeaway from FDA Imaging Forum: Need for Consensus Standards for Optical Coherence Tomography
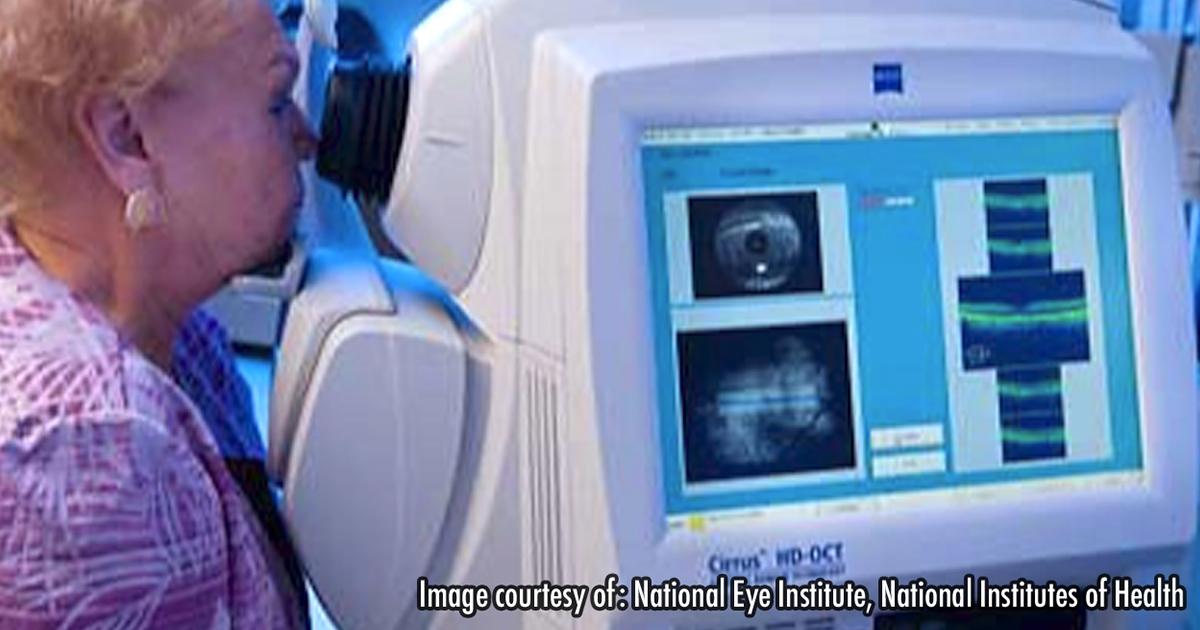
Continued advances in optical coherence tomography are generating excitement for more precise and earlier diagnosis of eye disease, but more research and industry agreement on standards are needed, researchers and leading ophthalmologists told participants at a workshop convened by medical organizations and the Food and Drug Administration.
OCT innovations were a primary point of discussion at the Forum on Laser-Based Imaging, a meeting held April 8 in Silver Spring, MD, and organized by the American Academy of Ophthalmology, America Glaucoma Society, American Society of Cataract and Refractive Surgery, American Society of Retina Specialists, Byers Eye Institute at Stanford University, and FDA, among others, to talk about the latest trends and technology enhancements in ophthalmic imaging.
Panelists concurred that advances in OCT imaging, such as OCT angiography, are enabling researchers to see more details of the eye’s basic structures, the quality of blood flow in the vessels of the eye, the levels of oxygen in the eye, the layers of cells in the retina, and even individual cells.
Need for Industry Standards
Presenters discussed that advances in OCT may result in replacing current diagnostic tools, such as fluorescein angiography, which is today’s standard for vascular imaging. OCT advances might also be able to help physicians diagnose eye disease more precisely and earlier than before.
The pathway for the FDA to determine if these new applications of OCT are as good as or better than current tools is rigorous. The challenge, however, is that there isn’t yet complete industry agreement on a “gold standard” for determining a glaucoma diagnosis, for measuring oxygen in the eye, for assessing retinal vascularity, or for assessing blood flow. Without that gold standard, it is difficult for physicians and regulators to assess just how much better advances in OCT are than existing technologies.
“I think what we heard was a general consensus that there is a need for very precise standards against which we can judge the new technologies coming along,” said Mark S. Blumenkranz, MD, H.J. Smead Professor emeritus and director of the Ophthalmic Innovation Program at Stanford’s Byers Eye Institute from 2015 to 2017.
Dr. Blumenkranz moderated a nine-person panel discussion among ophthalmologists working with OCT to provide further insight on its promise and challenges.
OCT Sheds New Insights in Glaucoma
Speaking prior to Dr. Blumenkranz’s panel convening, Richard Spaide, MD, a retina specialist with Vitreous Retina Macula Consultants of New York, highlighted how OCT innovation has enabled researchers to examine in great detail the blood vessels and nerve cells in the eyes of patients with glaucoma. It has resulted in speculation that glaucoma might be related in some instances to diseases of the brain.
When looking at OCT images of glaucoma patients, researchers can see a loss of nerve cells and changes to blood vessels. The type of nerve cell loss in glaucoma can also be found in neurodegenerative diseases such as Alzheimer’s, dementia, and Parkinson’s.
“It raises the idea that glaucoma might be a neurovascular disease,” Dr. Spaide said. He added that examining the neurodegenerative aspect of eye disease could be a fruitful field for future research.
Where Further Work Is Needed
Among those who spoke about the promise and need for further work was Nadia Waheed, MD, MPH, associate professor of ophthalmology at Tufts University School of Medicine, Boston. She said OCT angiography provides a high-resolution window into the retina’s vascular network, but that the technology still can’t do everything that fluorescein imaging can.
Lama Al-Aswad, MD, MPH, associate professor of ophthalmology at Columbia University Medical Center, New York, raised the issue that no current agreement exists on how to diagnose glaucoma, which has resulted in over-diagnoses, under-diagnoses, and misdiagnoses. Determining how OCT will improve glaucoma diagnosis, therefore, needs further research and development including consensus standards.
FDA’s Plan to Facilitate OCT Innovation
Brad Cunningham, MSE, RAC, an FDA branch chief, supervisory biomedical engineer, and acting associate director of the Office of Health Technology at the U.S. Public Health Services’ Center for Devices and Radiological Health, also took part in the panel discussion.
Dr. Cunningham said his job was to listen to panelists’ concerns and he encouraged panelists and audience members to engage with the FDA to help the agency early on, to “bring these technologies to market faster in a more meaningful way.”
At the very beginning of the conference, Dr. Cunningham gave a presentation laying out the FDA’s regulatory approval process for new devices. He also outlined the agency’s pilot program for speeding up the review process for innovations to OCT devices. The goal is to facilitate innovation of laser-based imagine modalities, he said.
For questions about this article, contact bara.vaida@gmail.com.
