OIS@AAO
Two principals of the alliance between the American Academy of Ophthalmology and Digisight share the genesis of the deal that will deliver insightful data to…
Read MoreCEO David Parke II, MD, explains why the American Academy of Ophthalmology continues to emphasize innovation, including the creation of the valuable ISIS patient registry.
Read MoreBrent Saunders, chairman, president, and CEO, says Allergan has no plans to exit ophthalmology. The pending loss of Restasis will require some cuts, but the…
Read MoreThe OIS Index is an ophthalmic industry stock index, launched at OIS@AAO in October 2016, as a tool to track the investment performance of publicly…
Read MoreRE-VANA Therapeutics, fresh off a US $1.6 million (£1.2 million) round of seed funding in 2017, is focusing preclinical investigation of its sustained-release drug-delivery technology…
Read MoreFive years out from Food and Drug Administration approval of its flagship iStent micro-invasive insert for treatment of glaucoma, Glaukos is now transitioning into a…
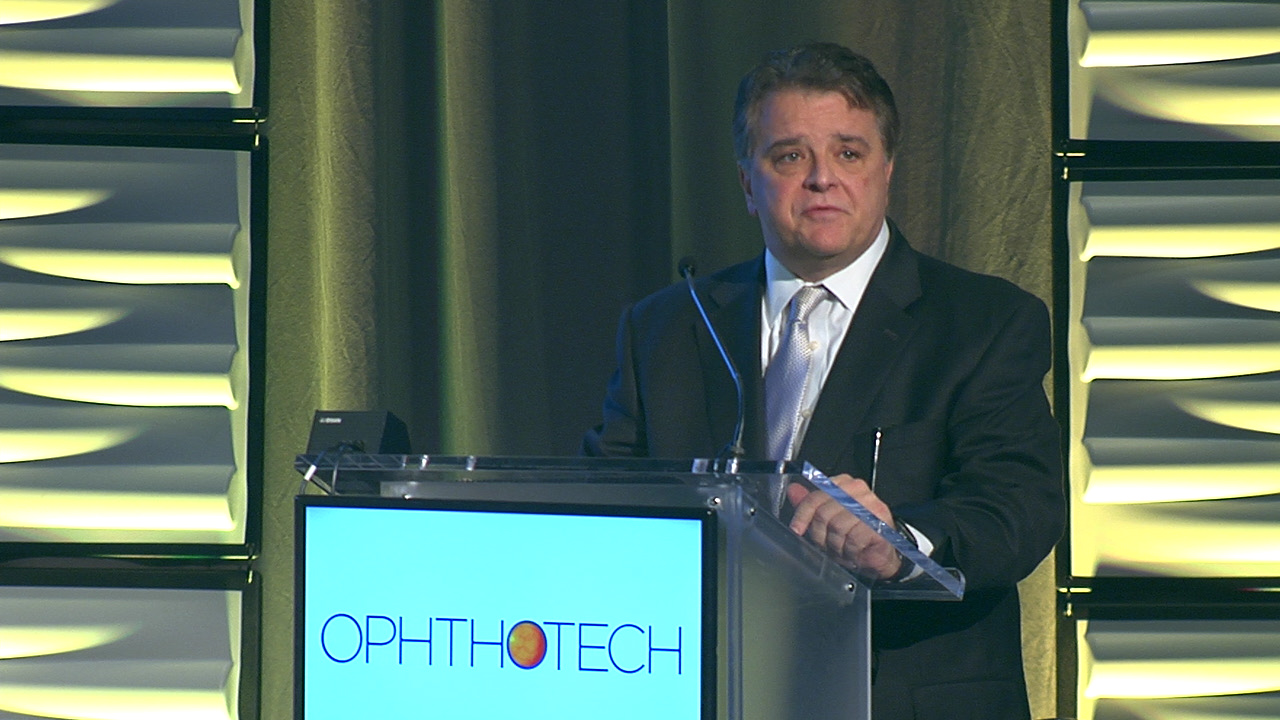
Read MoreOphthotech Corporation expects to start new clinical trials of its Zimura C5 inhibitor for three new indications within the next year, CEO and president Glenn…
Read MoreKala Pharmaceuticals is expecting by the end of 2017 topline clinical data from its ongoing Phase III trial of KPI-121 0.25% for treatment of dry…
Read MoreClearside Biomedical has a full agenda of major near-term milestones in the development of its suprachoroidal CLS-TA (triamcinolone-acetonide) platform in 2018 and 2019, president and…
Read MoreAlimera Sciences’ Iluvien insert for treatment of diabetic macular edema is making headway in the market, CEO Dan Myers said at the Company Showcase 3…
Read MoreEven though its PDUFA date for the glaucoma agent Rhopressa is set for February 2018, Aerie Pharmaceuticals is moving forward on development of its age-related…
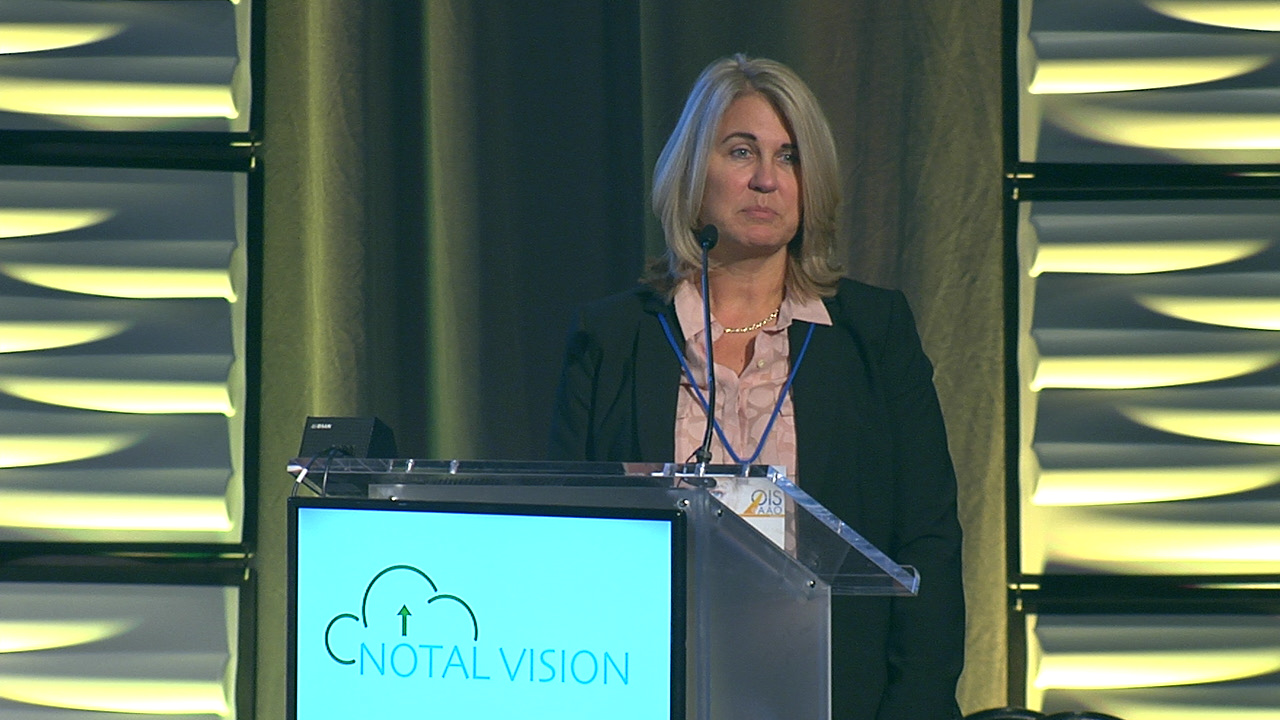
Read MoreNotal Vision is set to launch its second product, home-based optical coherence tomography (OTC) for monitoring of retinal fluid accumulation in patients with retinal disease,…
Read MoreSUBSCRIBE TO OIS NEWS
Get Our Weekly Newsletter About The Latest In Ophthalmic Innovation.
We respect your privacy and promise to never send you spam.












![OIS-ANTERIOR-NEW-DATE-750x420[4300] OIS-ANTERIOR-NEW-DATE-750x420[4300]](https://ois.net/wp-content/uploads/2020/03/OIS-ANTERIOR-NEW-DATE-750x4204300.jpg)